HIMS Halts Copycat GLP-1 Pill After FDA Warns Of "Swift Action"
Update (Saturday):
The official Hims & Hers Health X account, "Hims & Hers Comms," revealed in the early afternoon (a very convenient time to drop news) that its newly launched $49 a month copycat GLP-1 pill, positioned against Novo Nordisk's $149 a month Wegovy pill, will no longer be offered.
*HIMS & HERS TO STOP OFFERING WEIGHT LOSS PILL https://t.co/tiQoqBHf0K
— zerohedge (@zerohedge) February 7, 2026
"Since launching the compounded semaglutide pill on our platform, we've had constructive conversations with stakeholders across the industry," HIMS wrote on X.
It continued, "As a result, we have decided to stop offering access to this treatment. We remain committed to the millions of Americans who depend on us for access to safe, affordable, and personalized care."
Since launching the compounded semaglutide pill on our platform, we’ve had constructive conversations with stakeholders across the industry. As a result, we have decided to stop offering access to this treatment. We remain committed to the millions of Americans who depend on us…
— Hims & Hers Comms (@HimsHersComms) February 7, 2026
HIMS' decision to pull the $49 a month copycat GLP-1 pill comes about two days after FDA Commissioner Marty Makary warned against companies "mass-marketing illegal copycat drugs, claiming they are similar to FDA-approved products."
Makary's statement was directly pointed at HIMS.
FDA will take swift action against companies mass-marketing illegal copycat drugs, claiming they are similar to FDA-approved products.
— Dr. Marty Makary (@DrMakaryFDA) February 5, 2026
The FDA cannot verify the quality, safety, or effectiveness of non-approved drugs.
For HIMS bulls, brace for Monday, shaping up to be one of those gap-down mornings...
* * *
Hims & Hers Health's $49-a-month copycat GLP-1 pill, priced far below Novo Nordisk's $149-a-month Wegovy pill, can be seen as part of a "GLP-1 price war" between the telehealth company and Big Pharma giant.
It appears that HIMS' strategy has been an access-and-pricing arbitrage play: mass-market a copycat GLP-1 first, then address any regulatory fallout later.
In pure 'FAFO' fashion, HIMS is finding out very fast: hours after Thursday's press release touting its new GLP-1 pill for $49 per month to take on Wegovy, FDA Commissioner Marty Makary wrote on X that his agency will take "swift action against companies mass-marketing illegal copycat drugs, claiming they are similar to FDA-approved products."
Makary noted, "The FDA cannot verify the quality, safety, or effectiveness of non-approved drugs."
FDA will take swift action against companies mass-marketing illegal copycat drugs, claiming they are similar to FDA-approved products.
— Dr. Marty Makary (@DrMakaryFDA) February 5, 2026
The FDA cannot verify the quality, safety, or effectiveness of non-approved drugs.
For context, last June, Novo terminated its partnership, citing the telehealth company's "illegal mass compounding and deceptive marketing."
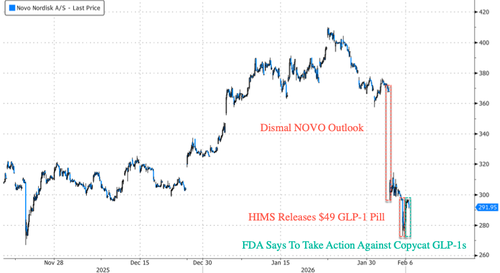
On Tuesday, Novo reported a disappointing full-year outlook, warning of a tough year in the GLP-1 market. Besides HIMS, the company faces competition from Eli Lilly's Zepbound, gaining ever-larger market share in the U.S.
Makary's comments on X sent HIMS shares down about 7.5% in premarket trading in New York. Shares of Novo in Europe are up about 4.5%.
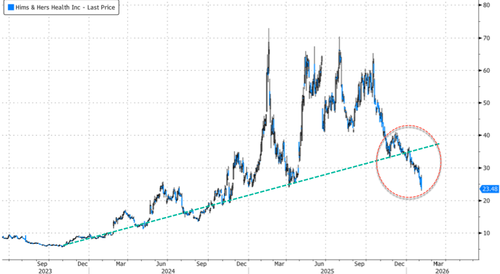
HIMS bubble unwinds...
We must also note that Makary's swift comments about copycat GLP-1 drugs were likely a nudge from Novo, as shares have been obliterated this week.
Goldman's Novo superbull James Quigley stated earlier this week, "FY26 is a reset year with respect to the pricing aspect of the GLP-1 market."